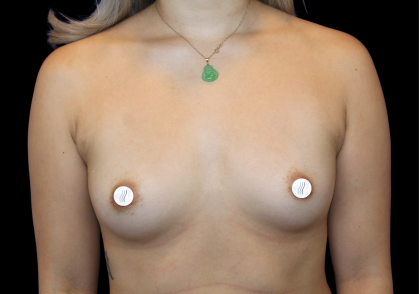
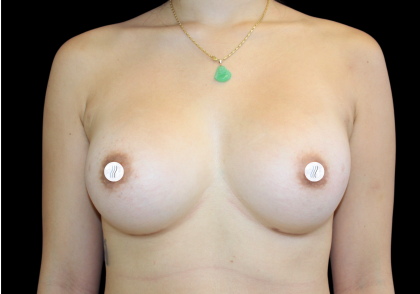
Breast Implant fill – Saline or Silicone?
Conveniently located to serve the areas of Honolulu, HI
Saline and silicone implants are two common options for breast augmentation. The first implants were filled with silicone gel. As breast implants evolved, saline (salt water) became an accepted alternative fill to silicone, although silicone can look and feel more like a real breast. Whether filled with silicone gel or salt water, the shell of both types is made of a firmer, silicone elastomer.
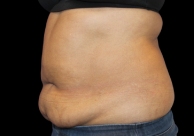
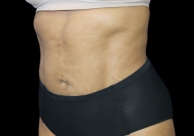
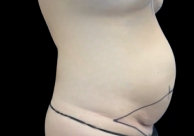
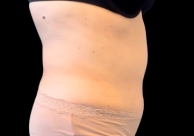
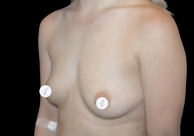
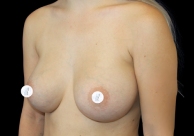
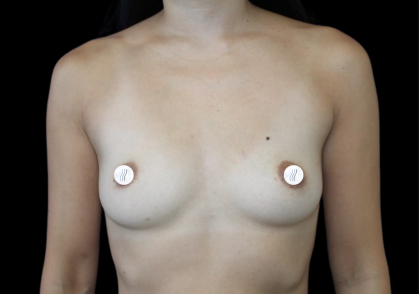
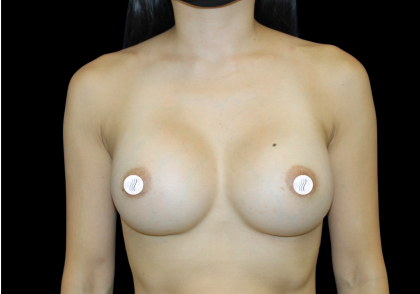
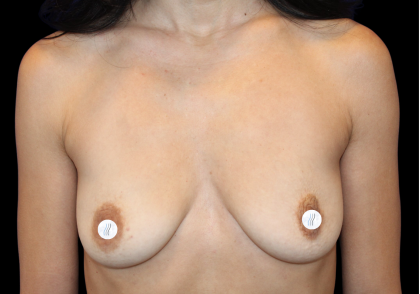
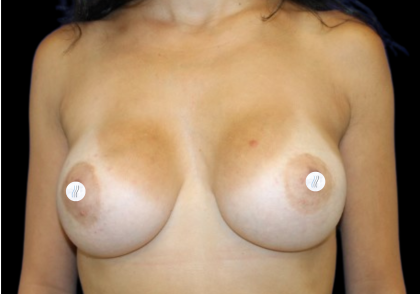
Before and After Photos
Read more about saline breast implants
Read more about silicone gel breast implants